Epoxy Vinyl Ester Resins (VER)
(Acrylated Epoxy Resins)
Properties
Epoxy vinyl ester resins (VER), Often shortened to vinyl esters, are an important class of high-performance thermoset molding resins. They are produced by the addition of α - β unsaturated carboxylic acids to epoxy resins. The two main types of epoxy resins are bisphenol A diglycidyl ether (DGEBA) and epoxy phenol novolac (EPN). These resins form durable laminates and coatings when fully cured (cross-linked). Their performance is often between those of conventional unsaturated polyesters and epoxy resins.
The properties of the cross-linked resin depend on the type of epoxy and vinyl monomer (reactive diluent) used and their relative amounts. The majority of commercial vinyl polyester resins are derived from bisphenol A diglycidyl ether (DGEBA) as the epoxy component and acrylic or methacrylic acid as the vinyl monomer in the polymer. Many other epoxies and vinyl acids or esters can be used to taylor the properties of unsaturated polyesters. For example, (modified) novolac epoxies are sometimes chosen to achieve better thermal and chemical resistance, whereas dimer fatty acid - glycidyl methacylate modified resins have improved flexibility but reduced chemical and heat resistance.
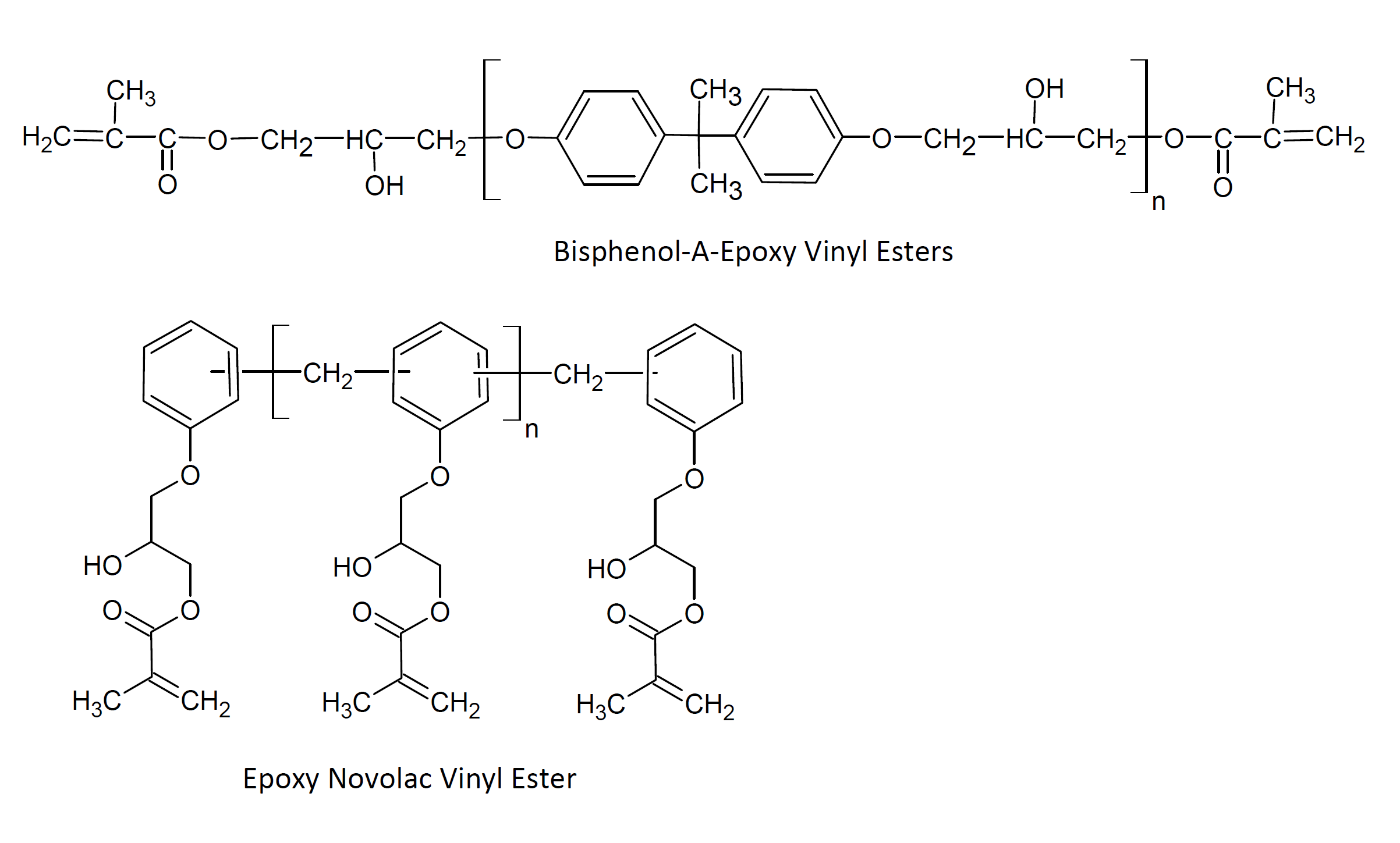
Vinyl ester resins are relative viscous low-molecular-weight prepolymers. To make them more workable, they are typically dissolved in a vinyl monomer (and occasionally a solvent1) together with an appropriate organic peroxide.2 The mixture is poured, sprayed, or molded into the final product and then converted into a thermoset by heating. Many resins can also be room temperature cured when a reducing agent such as cobalt naphthenate or octoate or an aromatic tertiary amine is added to the sytem. This compound is often called accelerator or activator. Resin products with an activator are typically two-pack systems whereas heat curable VERs are typically one-part systems. The cure time and pot-life will depend on the initiator system, its concentration as well as on the type of resin and vinyl monomer and cure temperature.
The most common vinyl monomer is styrene. It functions as a reactive diluent and as a crosslinking agent. When polymerized in the presence of unsaturated polyester, it reacts with the double bonds of the prepolymer but also undergoes homopolymerization. The degree of homo- and co-polymerization depends on the reaction conditions, monomer content, and miscibility of the two resins.
Due to growing health concern3, the styrene content has been greatly reduced in many resin systems or completely replaced by less toxic vinyl monomers such as vinyl toluene, vinyl acetate, or methyl (meth)acrylate as well as by difunctional vinyl monmers such as diacrylates and dimethacrylates. The type of reactive diluent will effect both the cure and the final properties of the polyester.
Some VERs can also be chemically modified to achieve workable viscosities without the addition of reactive diluents. For example, the reaction of secondary hydroxyl groups of the epoxy part with diisocyanate greatly lowers the viscosity of the resin.5
Commercial Epoxy Vinyl esters
Epoxy vinyl ester resins are produced on a large scale by a number of companies including Ashland, Reichhold, and Interplastic. They are typically sold in liquid form and are often blended with reactive diluents (styrene) to reduce their viscosity. Grades with different viscosities, epoxies and gel time are available.
APPLICATIONS
VERs are sometimes used in place of conventional unsaturated polyester resins (UPEs). They are typically more expensive than UPE resins but are still widely used, because they have superior chemical and heat resistance, lower water absorption, and superior hydrolytic stability. Thus, VERs are more suitable for aggressive environments than UPEs. Important applications include the production of fiber reinforced plastics and filled plastic products such as solvent tanks, sewer pipes, and structural components for the marine and transportation industry. Epoxy vinyl ester resins also find uses in coatings, particulalry for the marine, building and construction industries due to their excellent corrosion and weathering resistance.
References and Notes
-
In some cases, conventional solvents such as xylenes are also used. However, most are prepared solely with reactive vinly type monmomers.
-
Common initiators include methyl ethyl ketone peroxide (MEKP), benzoyl peroxide (BPO), and acetylacetone peroxide (AAP).
-
Acute (short-term) exposure to styrene can cause mucous membrane, skin, and eye irritation, whereas chronic (long-term) exposure can effect the central nervous system (CNS) resulting in headache, fatigue, weakness, etc. Styrene is also suspected to cause cancer.5
-
The Department of Occupational Medicine at Aarhus University in Denmark concluded that styrene may cause cancer in humans.
-
S. Jaswal and B. Gaur, Reviews in Chemical Engineering, Vol. 30, 6, pp. 567 (2014)